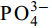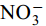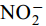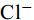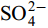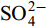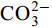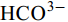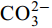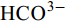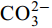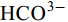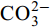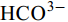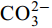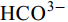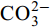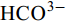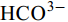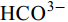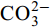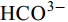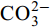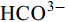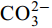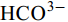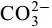PREPARATION OF BIOCHAR BY LOW TEMPERATURE PYROLYSIS OF LANTHANUM MODIFIED SHRIMP SHELLS FOR EFFICIENT PHOSPHORUS ADSORPTION
-
摘要:
为提高生物炭对磷的去除效果和将厨余废弃物的资源化利用, 文章以废弃虾壳为原料, 用NaOH将LaCl3以La(OH)3沉淀形式附着在虾壳表面, 进行热解得镧改性生物炭(CSLa)。采用XRF、SEM、BET、FTIR和XRD对改性前后的生物炭表征分析。采用吸附等温线模型和吸附动力学模型拟合生物炭的吸磷特征。研究了改性剂用量、初始pH、共存干扰离子对生物炭吸附磷的影响。结果表明镧化合物负载在生物炭表面, 对磷吸附能力明显提高, 最大理论吸附量为160.51 mg/g, 与CS400磷最大吸附量(100.60 mg/g)相比约提高60%。在低浓度或高浓度磷溶液条件下, CSLa对磷吸附量和去除率均高于CS400, 在实际水产养殖废水中更实用。吸附过程主要受化学吸附、颗粒内扩散控制。有关机理分析的结论表明表面沉淀作用、静电吸引、配体交换和内层络合作用是CSLa吸附磷的主要机理。CSLa更适合在弱酸性环境中除磷, 不过在碱性环境条件下其吸附量也比较高。
${\rm{HCO}}^-_3 $ 和${\rm{CO}}^{2-}_3 $ 对CSLa磷吸附有一定的抑制作用。研究以低温400℃为热解温度制备镧改性生物炭, 不仅提升了生物炭对磷的吸附量和去除率, 而且消耗热能较少, 更有利于实际应用。Abstract:In order to improve the phosphorus removal effect of biochar and resource utilization of food waste, we obtained lanthanum-modified biochar (CSLa) by using waste shrimp shells as raw material and attaching LaCl3 to the surface of shrimp shells in the form of La(OH)3 precipitation with NaOH by pyrolysis. The modified and unmodified biochar samples were characterized and analyzed by XRF, SEM, BET, FTIR and XRD. The adsorption kinetic and adsorption isotherm models were used to fit the phosphorus absorption characteristics of the biochar. The effects of modifier dosage, initial pH and coexisting interfering ions on phosphorus adsorption by biochar were investigated. The results showed that lanthanum modified shrimp shell biochar (CSLa) had abundant oxygen-containing functional groups on its surface. CSLa consisted mainly of La2O3, and its pore size distribution was dominated by mesoporous pores. The phosphorus adsorption process was better fitted with Langmuir isotherm, indicating the adsorption of phosphorus on CSLa was monolayer adsorption. The adsorption of phosphorus on CSLa was better described by quasi-secondary kinetics, indicating the phosphorus adsorption process by CSLa was chemisorption. The maximum theoretical adsorption capacity of CSLa was found to be 160.51 mg/g, achieved after 12h of adsorption. The adsorption and removal rates of phosphorus by CSLa were higher than those by CS400 at low or high phosphorus concentrations, which was more practical in real aquaculture tailwater. The adsorption rate of phosphorus on CSLa was mainly controlled by chemical adsorption and intraparticle diffusion. The mechanism analysis indicated that surface precipitation, electrostatic attraction, ligand exchange and internal complexation were the main mechanisms of phosphorus adsorption by CSLa. CSLa demonstrated better phosphorus removal performance under weakly acidic conditions, while the adsorption capacity was relatively high under alkaline environmental conditions. The interfering ions Cl–,
${\rm{NO}}^-_3 $ ,${\rm{NO}}^-_2 $ and${\rm{SO}}^{2-}_4 $ had no effect on CSLa phosphorus adsorption, while${\rm{HCO}}^-_3 $ and${\rm{CO}}^{2-}_3 $ had some inhibitory effect on CS400 phosphorus adsorption. In this study, lanthanum-modified biochar was prepared at a low temperature of 400℃ as the pyrolysis temperature, which not only enhanced the adsorption and removal rate of phosphorus by biochar, but also consumed less thermal energy, which was more beneficial to practical applications.-
Keywords:
- Shrimp shell biochar /
- Phosphate adsorption /
- Lanthanum modification
-
我国池塘养殖主要以淡水养殖为主, 内源污染中的氮磷污染, 严重影响水产品质量和产量[1-3], 必须净化养殖水体中的氮磷。对于脱氮过程, 水产养殖业已经建立了相当成熟的技术和工艺, 而对于脱磷技术却很少有人进行研究[4, 5]。
为了解决磷污染问题, 需要寻找从废水中高效去除并回收磷酸盐的方法[6]。吸附法因其成本廉价、高效快捷、设备简单、运行可靠等优点被认为是最有效的除磷方法[7, 8]。生物炭吸附材料因廉价易得, 官能团丰富、比表面积大, 结构多孔及较高的环境稳定性而受到青睐[9, 10]。但在生物炭制备过程中, 不可避免需要较高热能来热解吸附材料制备生物炭[11], 为降低能耗本研究试图以低温热解吸附材料制备生物炭, 既得到高效的磷吸附材料又节约电能。
克氏原螯虾(俗称小龙虾)是重要的食用水产品, 每年人类消耗的小龙虾高达数万吨, 产生了大量的虾壳废弃物。本研究以废弃虾壳为原料制备生物炭, 通过镧改性虾壳生物炭, 以低温制备高性能的磷吸附材料, 对改性前后的生物炭表征分析。采用吸附动力学模型和吸附等温线模型拟合生物炭的吸磷特征。研究了单因素对生物炭吸附磷的影响, 考察生物炭的回收与再利用性能。
1. 材料与方法
1.1 虾壳生物炭的制备与改性
本文选取的虾壳原料来自江苏连云港, 将虾壳进行清洗, 烘干, 研磨, 储存至干燥器中备用。
虾壳生物炭的制备 将虾壳粉在氮气氛围下管式炉中热解, 设置热解温度400℃, 升温速率10℃/min, 热解时间3h (热解条件依据前期预实验所得), 将生物炭过100目筛后反复清洗至干净, 烘干, 装入自封袋中备用。400℃制得虾壳生物炭简写为CS400, 吸附磷饱和CS400简写为CS400-P。
La(氯化镧)改性虾壳生物炭的制备 参考以往镧改性生物炭的研究[12-14], 取10 g虾壳粉, 按La/虾壳为0、0.002、0.004、0.006、0.008、0.01、0.012、0.014、0.016和0.018 mol/g溶于100 mL蒸馏水中, 超声, 搅拌, 缓慢滴加足量1 mol/L的NaOH溶液, 然后清洗至pH为7, 烘干, 研磨, 热解, 热解参数和虾壳生物炭制备一致, La改性的虾壳生物炭简写为CSLa, 吸附磷饱和的CSLa简写为CSLa-P。
1.2 生物炭表征
采用扫描电子显微镜 (SEM, SU8010, 日本; Sigma500, 德国蔡司(仅用于扫描CSLA)观察生物炭的表面形貌结构; 采用 Zeta 电位分析仪 (Zeta, Malvern Zetasizer Nano ZS90, 英国) 测定生物炭表面电荷; 采用全自动比表面积和孔隙度测定仪 (BET, ASAP 2020, 美国) 测定生物炭的比表面积大小和孔径分布; 采用X 射线衍射仪 (XRD, D/Max 2500, 日本) 分析晶体类型; 采用傅里叶红外光谱仪 (FT-IR, Nicolet iS10, 德国) 分析生物炭的表面官能团; 采用X射线荧光(XRF, RIGAKU ZSX Priums, 日本)分析生物炭的成分组成。
1.3 影响因素
通过控制改性剂用量、溶液初始pH和干扰离子探究各因素对CS400和CSLa吸附磷的影响。溶液的初始pH用HCl(0.1 mol/L)和NaOH(0.1 mol/L)溶液控制。
1.4 吸附等温线
分别加入0.01 g CS400/CSLa于50 mL锥形瓶中, 依次加入20 mL不同初始浓度(30、60、120、180、240和300 mg/L)的磷溶液(pH=5), 设置实验温度为15℃、25℃和35℃, 转速180 r/min, 振荡48h后取上清液过0.45 µm针孔滤膜测定滤液浓度。采用 Langmuir (式1)和Freundlich (式2)对吸附等温线进行拟合和分析; 同时增加CS400/CSLa在25℃时对不同初始低浓度(0.6、1.2、3、6、12、20和30 mg/L)的磷溶液(pH=5)的吸附量, 其他条件与吸附等温实验相同。
$$ {\rm{Langmuir}} : \frac{{\text{C}}_{\text{e}}}{{\text{Q}}_{\text{e}}}\text=\frac{\text{1}}{{\text{k}}_{\text{1}}{\text{Q}}_{\text{m}}}\text+\frac{{\text{C}}_{\text{e}}}{{\text{Q}}_{\text{m}}} $$ (1) $$ {\rm{Freundlich}} : \text{ln}{\text{Q}}_{\text{e}}\text{=ln}{\text{k}}_{\text{f}}+\text{nln}{\text{C}}_{\text{e}} $$ (2) 式中, Ce为达到吸附平衡时溶液中剩余的磷酸盐浓度, mg/L; Qe为达到吸附平衡时的吸附量, mg/g; k1为Langmuir常数, L/mg; kf为Freundlich常数, (mg/g)·(L/mg)1/n。
1.5 吸附动力学
分别加入0.01 g CS400/CSLa于不同50 mL锥形瓶中, 依次加入20 mL初始浓度为100 mg/L的磷溶液(pH=5), 25℃、转速180 r/min下振荡, 在不同时间(0.5—48h)取上清液过0.45 µm针孔滤膜测定滤液浓度。采用准一级动力学模型(式3), 准二级动力学模型(式4), Elovich 模型(式5)和颗粒内扩散动力学模型(式6)[15-17]对吸附结果进行拟合。
$$ 准\, 一\, 级\, 动\, 力\, 学:{\text{Q}}_{{t}}\text={\text{Q}}_{\text{m}}\left({1-}\frac{1}{{\text{e}}^{-{\text{k}}_{\text{1}}{t}}}\right) $$ (3) $$ 准\, 二\, 级\, 动\, 力\, 学: \frac{{t}}{{\text{Q}}_{{t}}}\text=\frac{\text{1}}{{\text{k}}_{\text{2}}{\text{Q}}_{\text{m}}^{\text{2}}}\text+\frac{{t}}{{\text{Q}}_{\text{m}}} \quad $$ (4) $$ {\rm{Elovich}} : {\text{Q}}_{\text{t}}\text=\frac{\text{1}}{\beta}\text{ln}\left(\text{\alpha \beta}\right)\text+\frac{\text{1}}{\beta}\text{ln}{(}{t}{)} \quad$$ (5) $$ 颗\, 粒\, 内\, 扩\, 散\, 动\, 力\, 学: {\text{Q}}_{\text{t}}\text={\text{k}}_{\text{w}}{{t}}^{\text{0.5}}\text{+b}\quad $$ (6) 式中, Qt为吸附时间为t时吸附量, mg/g; Qm为达到吸附平衡时的理论吸附量, mg/g; k1为准一级动力学模型的吸附速率常数, /min; k2为准二级动力学模型的吸附常数, g/(mg·min);
$\alpha $ 为初始生物炭吸附速率, mg/(g·min);$\beta $ 为Elovich模型解吸常数, mg/g; kw为颗粒内扩散动力学模型的常数, /min [18-21]。1.6 吸附剂的磷释放性能和磷的回收与利用研究
取0.25 g CS400/CSLa各自投加到500 mL烧杯中, 加入500 mL的KH2PO4溶液(浓度为100 mg/L, 以P的浓度计), 未进行调pH (pH为5), 在温度设置为25℃和转速设置为180 r/min的磁力搅拌器上, 反应时间48h后, 使用超纯水反复清洗生物炭表面吸附的溶解性磷, 用真空循环水泵过0.45 µm膜, 对生物炭进行收集, 在105℃恒温箱中干燥至恒重。
附剂的磷释放性能研究 取收集的生物炭0.01 g投加到50 mL锥形瓶中, 在每组锥形瓶中分别加入20 mL不同pH (3、4、5、6、7、8、9、10)纯水, 封盖, 在温度设置为25℃和转速设置为180 r/min的磁力搅拌器上, 反应时间48h后, 使用超纯水反复清洗生物炭表面吸附的溶解性磷, 取上清液过0.45 µm针孔滤膜测定滤液浓度。
磷的回收与利用实验 将收集的生物炭投加到500 mL的烧杯中, 加入500 mL的1 mol/L NaOH, 封盖, 放在磁力搅拌器上1h, 反应温度设为25℃, 转速为180 r/min, 使用超纯水反复清洗生物炭表面至中性, 在105℃恒温箱中干燥至恒重, 重复上述操作4次, 分别计算每次循环的吸附量。
1.7 吸附实验分析方法
采用钼酸铵分光光度法测定溶液中磷的浓度。根据 [式 (7)]计算平衡吸附量Qe (mg/g, 以 P 计)。
$$ {{Q}}_{\text{e}}\text={(}{{C}}_{\text{o}}-{{C}}_{\text{e}}\text{)}\frac{{V}}{{m}} $$ (7) 式中, Qe为吸附平衡后的P吸附量, mg/g; Co为初始溶液P浓度, mg/L; Ce为吸附平衡时溶液浓度, mg/L; V为溶液体积, L; m为生物炭用量, g。
2. 结果与讨论
2.1 改性生物炭的表征
XRF XRF (表 1)分析结果表明, CS400和CSLa的成分复杂, CS400的主要成分为CaO的相关化合物, 可与磷反应吸附磷, 这是CS400与其他生物炭相比磷吸附量高的主要原因。而CSLa的主要成分为La2O3和CaO的相关化合物, 表明镧成功负载在虾壳生物炭上, 提供更多的磷吸附位点, 这是CSLa吸附量高于CS400的原因。
表 1 CS400和CSLa的X荧光光谱Table 1. X-fluorescence spectrum of CS400 and CSLa组分
Component含量
Content (%)组分
Component含量
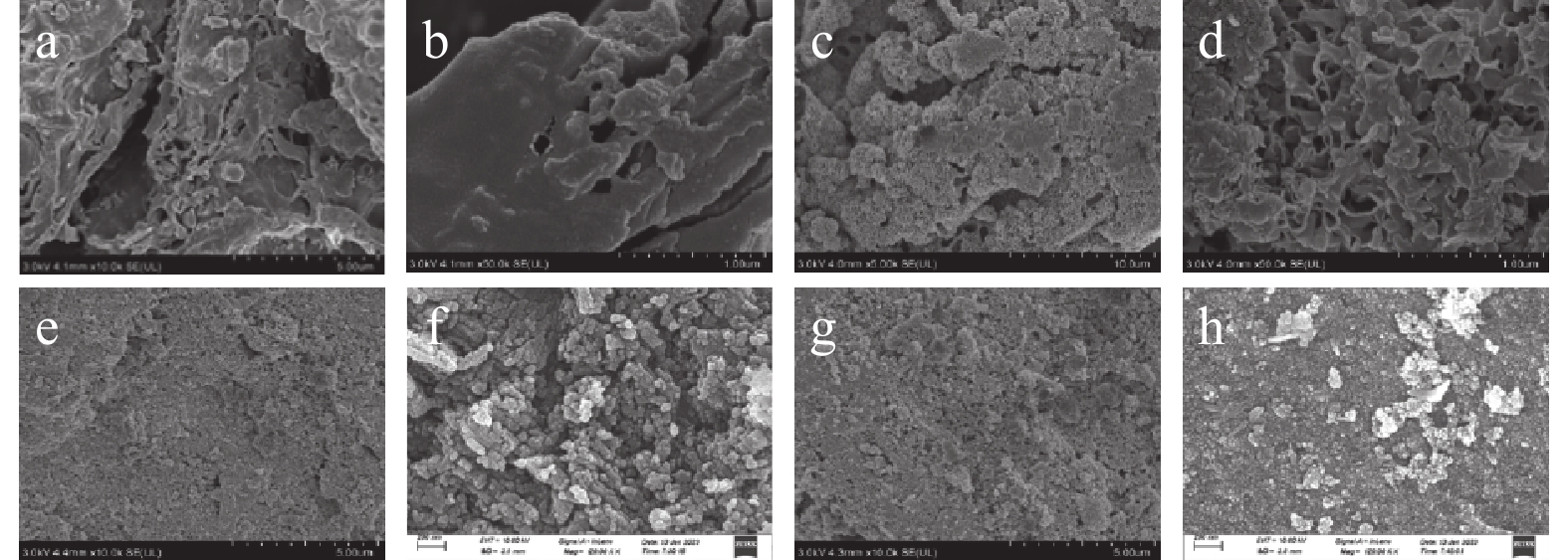
Content (%)CS400 CSLa CS400 CSLa La2O3 0.07 76.92 K2O 0.46 0.03 CaO 86.33 16.72 SrO 0.41 0.03 P2O5 5.74 1.28 TiO2 0.08 0.02 SiO2 2.28 0.41 ZnO 0.04 0.02 MgO 1.35 0.15 CuO 0.04 0.02 Fe2O3 0.82 0.25 ZrO2 0.02 / SO3 0.77 0.20 Cr2O3 0.01 / MnO 0.59 0.11 WO3 0.01 / Cl 0.51 2.85 NiO 0.01 0.01 Na2O / 0.40 PbO / 0.02 Al2O3 / 0.19 Tb4O7 / 0.07 Cs2O / 0.11 SrO / 0.03 CeO2 / 0.11 V2O5 / 0.03 SEM 图 1可知CS400表面平滑, 为不规则片状结构, 孔隙结构不发达。CS400-P表面较为粗糙, 也为不规则片状结构, 孔隙结构不发达, 表明P被吸附在CS400表面。
图 1可知CSLa表面粗糙, 为不规则块状结构, 孔隙结构不发达, 说明镧化物以颗粒状附着在虾壳生物炭表面。CSLa-P的颗粒聚集程度高于CSLa, 说明P被吸附在CSLa表面。
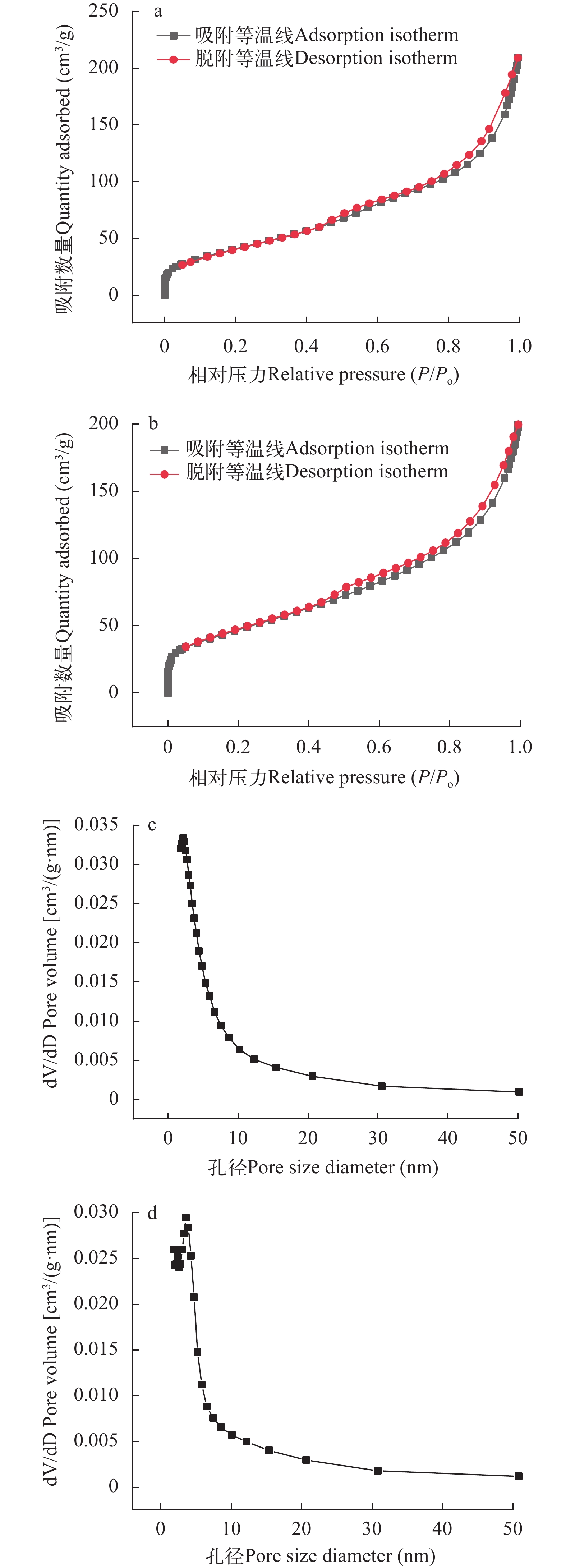
BET 表 2为生物炭的BET参数, 改性后的生物炭比表面积从170.06降至151.86 m2/g; 平均孔径由改性前的7.26增大至8.03 nm。这可能是因为CSLa表面和孔道中存在与镧相关的吸附位点, 部分的镧在负载时进入到了生物炭孔隙内部, 导致CSLa的平均孔径变大[22], 这也说明镧成功地负载在了虾壳生物炭上。图 2为N2吸附/脱附等温线和孔径分布曲线。CSLa和CS400的N2吸附脱附等温线介于Ⅱ和Ⅲ类N2吸附等温线之间。
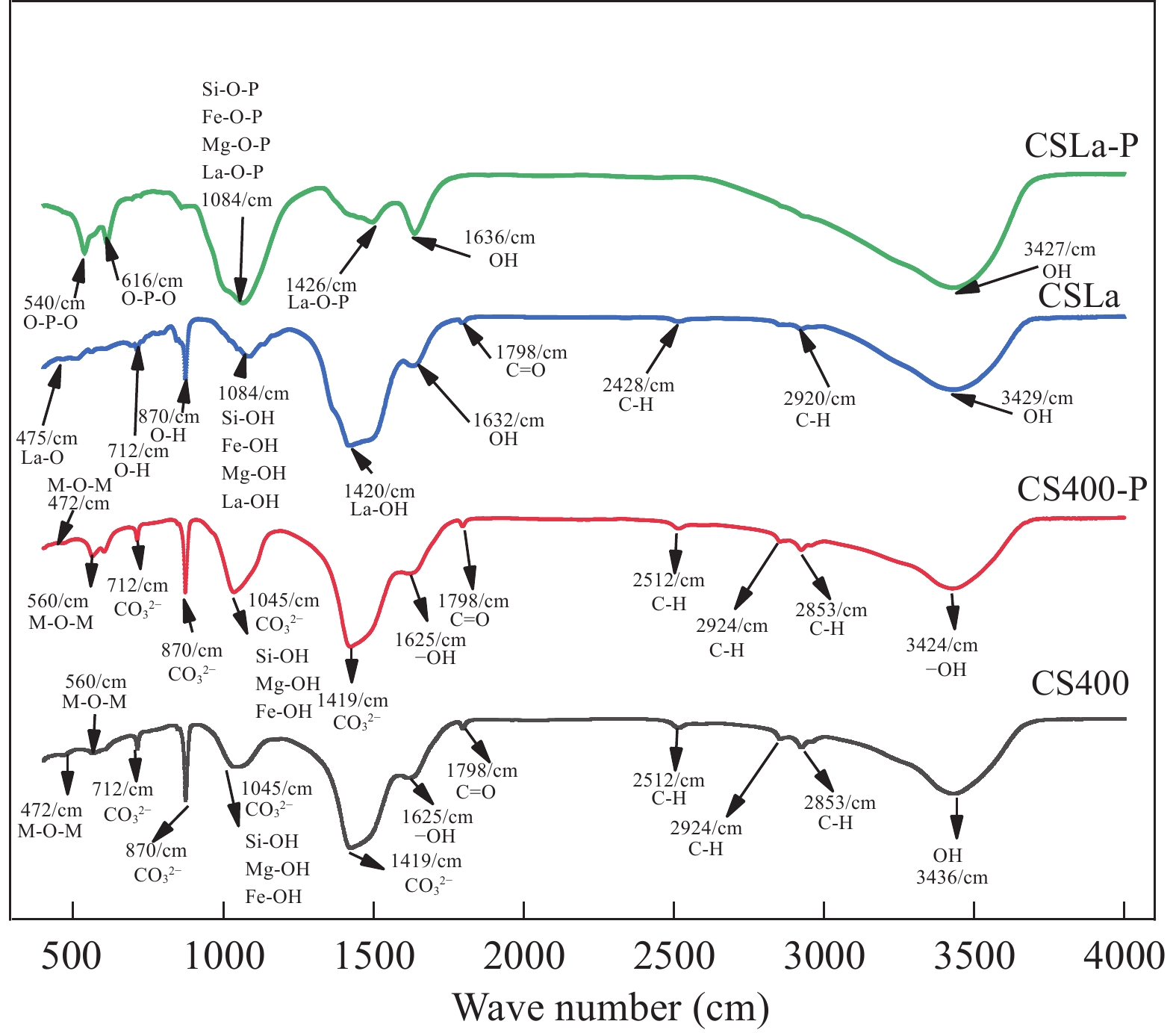
表 2 生物炭的BET参数Table 2. BET parameter of biochar样品Sample 表面积Specific area (m2/g) 体积Volume (cm3/g) 平均孔径Average pore diameter (nm) CS400 170.06 0.31 7.26 CSLa 151.86 0.30 8.03 FTIR 由CS400和CSLa的傅里叶变换红外光谱可知, CSLa-P的红外光谱在3434和1632/cm处的吸收峰强度明显增加(图 3), 说明镧改性增加了生物炭表面的O-H基团与磷酸盐发生了配位体交换作用生成复合物(La-O-P)[23]; CSLa-P的红外光谱中对应的特征峰明显减弱, 这可能是由于La-OH的-OH与磷酸盐酸根的内层络合[12]; CSLa在712和870/cm处的峰为La-OH中的-OH振动峰; 在1436/cm处的峰出现了一个新峰, 这是因为La(OH)3与空气中CO2反应形成的碳酸盐基团峰[24]; 在1084/cm处的强峰为M-OH振动峰, 与
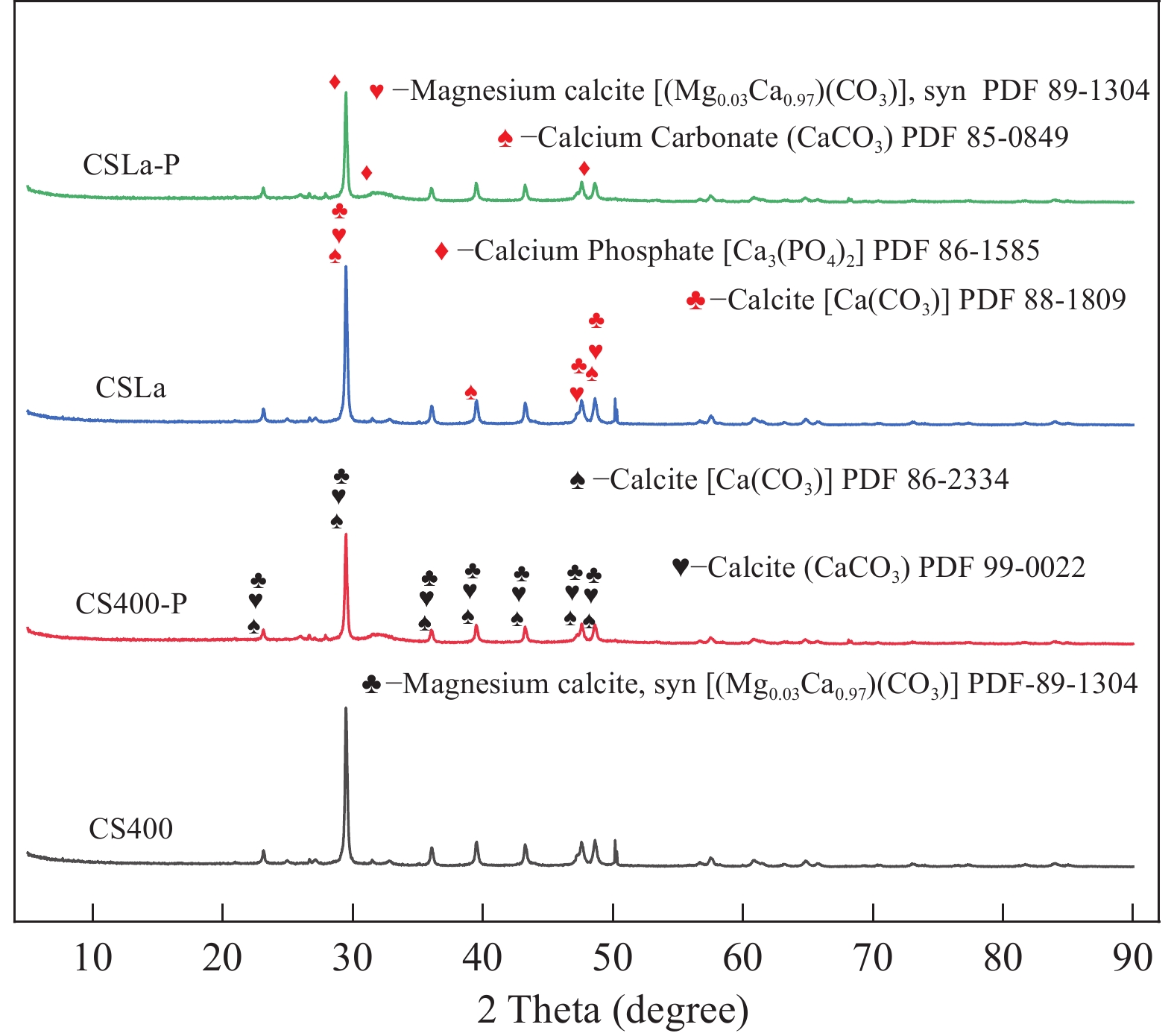
${\rm{PO}}^{3-}_4 $ 中P-O的不对称伸缩振动形成M-O-P[12], CSLa-P的红外光谱在540和616/cm处的峰对应为O-P-O的弯曲振动峰, 表明大量的磷酸盐成功吸附在CSLa上[25, 26]。XRD CS400的XRD图谱中(图 4), 可检索出方解石晶相(Calcite, CaCO3, JCPDS No.89-0022)和镁方解石晶相[Magnesium calcite, syn, (Mg0.03Ca0.97)(CO3), JCPDS No.89-1304]的特征峰,但其含量相当低。
CSLa的XRD(图 4)图谱中, 可检索出碳酸钙晶相[Calcium Carbonate (CaCO3), JCPDS No.85-0849], 方解石晶相[Calcium (CaCO3), JCPDS No.86-2340]和镁方解石晶相[Magnesium calcite, syn, (Mg0.03Ca0.97)(CO3), JCPDS No.89-1304]的特征峰。CSLa-P的XRD图谱中出现磷化钙晶相[Calcium Phosphide Ca3(PO4)2, JCPDS No.85-16585]但其含量相当低。这说明CS400和CSLa中磷的存在状态为无定形。
2.2 单因素影响研究
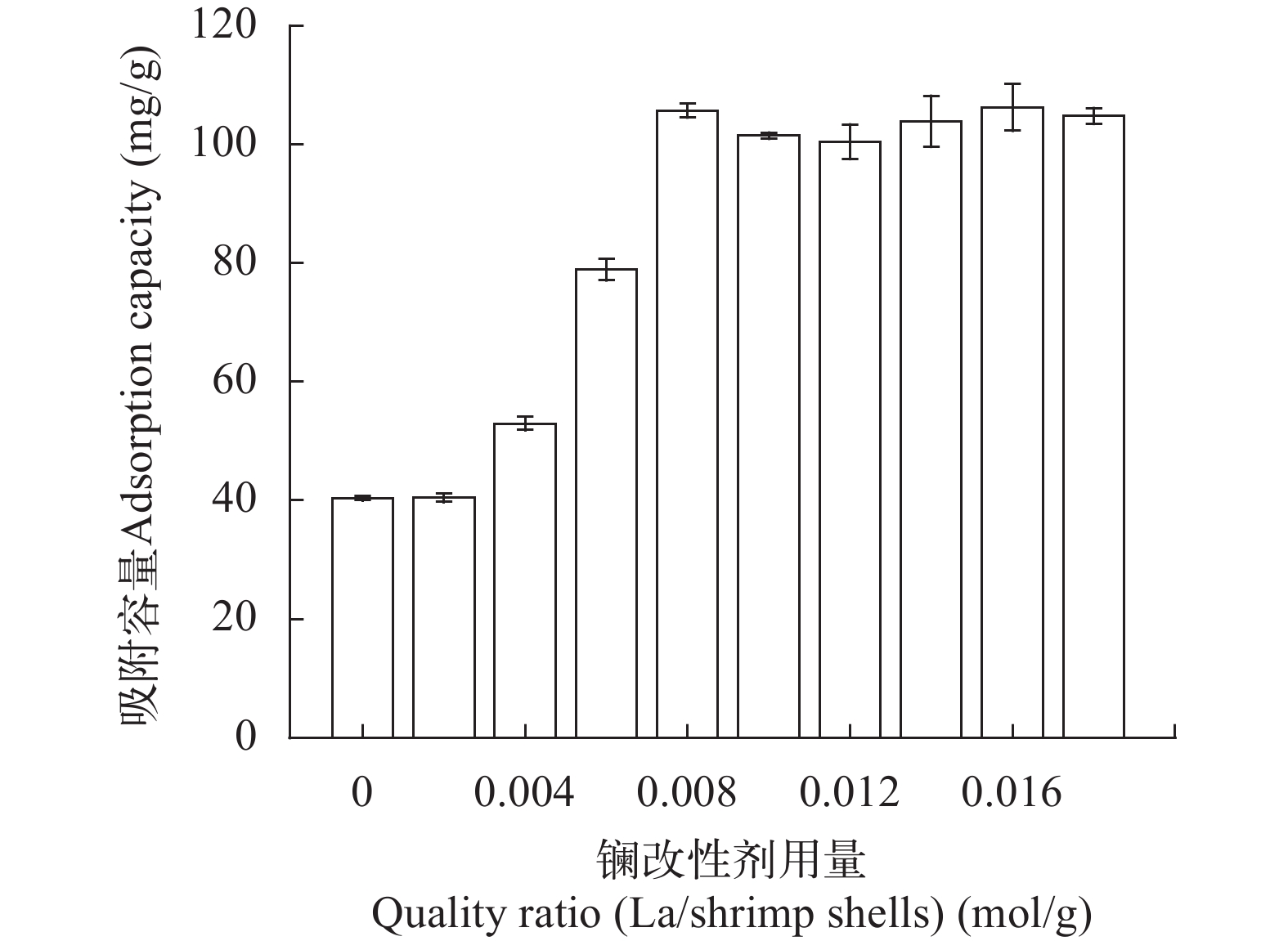
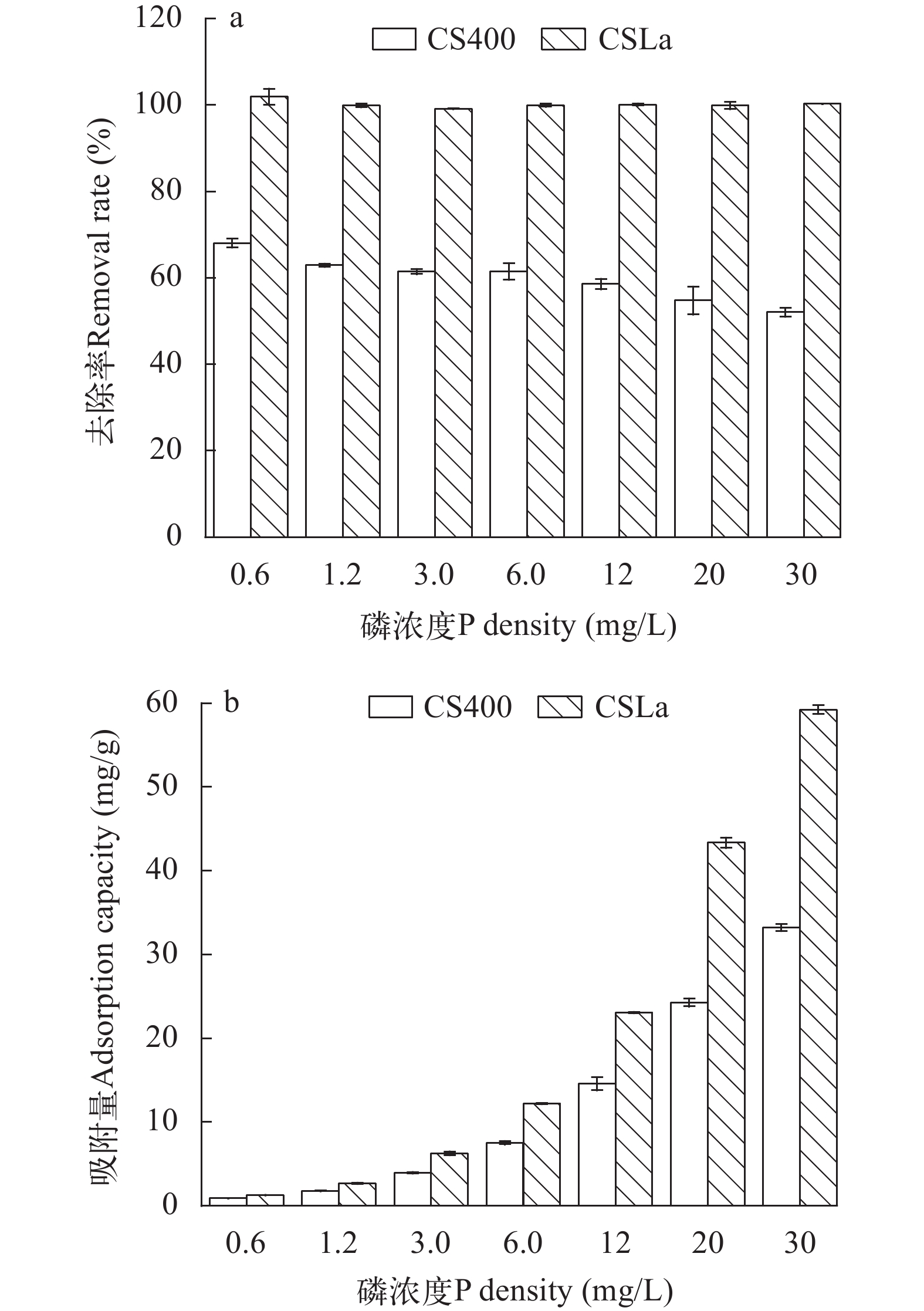
改性剂量对生物炭改性进而吸附磷酸盐的效果 在质量比(La/虾壳)为0.008 mol/g时, CSLa对磷酸盐的吸附量最高(105.70 mg/g), 远高于CS400对磷酸盐的吸附量(40.39 mg/g), 证明镧在生物炭表面的负载和孔隙的填充已达到饱和状态, 在所设计实验条件下磷酸盐吸附量也达到最大值(图 5)。
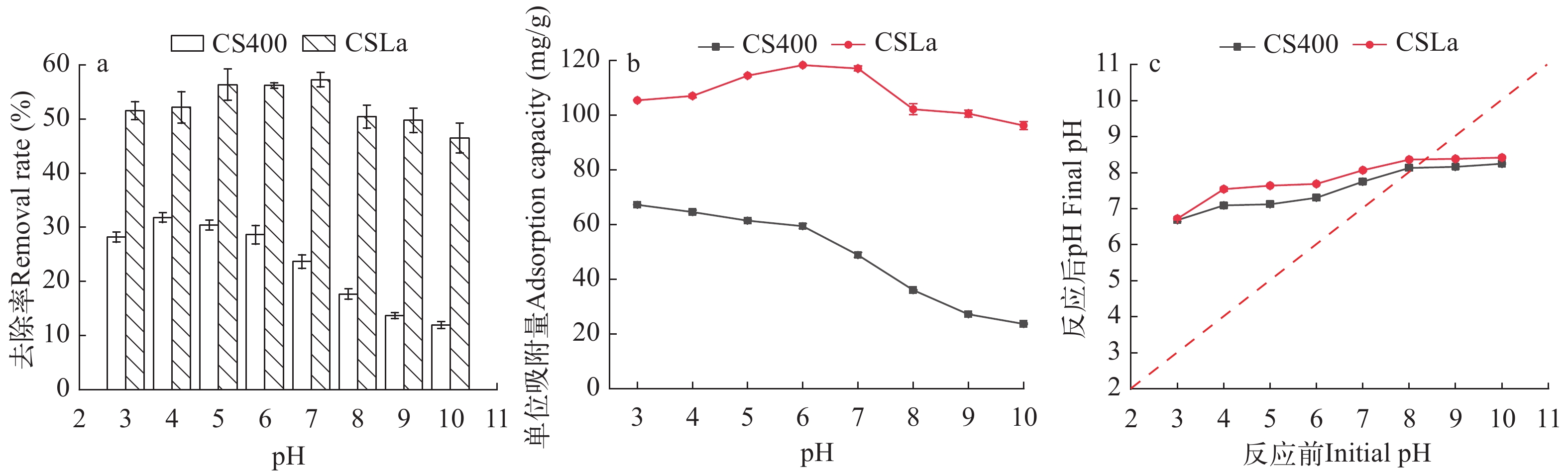
溶液初始 pH 对吸附效果的影响 由图 6可知CS400对磷酸盐吸附能力随pH的升高呈现逐渐减小的趋势, 而在pH为3时磷吸附量低于在pH为4时, 可能是由于酸性过强导致CS400中含有的磷酸盐释放的原因[27]。而对于CSLa对磷吸附能力随pH的升高呈现先增加后减少的趋势, 其间磷的吸附量在pH为7时最大, 这与以往有关生物炭吸附磷的研究相一致。CSLa在酸性环境对磷的吸附量高于碱性环境, 但其去除率仍能达到46%, 可知CSLa与CS400相比, 对磷的吸附量受溶液pH影响较小, 更适用于各种酸碱环境条件下的磷吸附。CSLa与CS400, pH在3—8时, 磷吸附后的溶液pH均小于初始溶液pH, 表面的羟基与溶液中的磷酸盐发生配体交换作用导致OH–释放从而使得溶液pH略有升高; 当初始pH为9或10时则相反, 说明当溶液pH较高时, 不利于配体交换反应的发生, 这一结论和Li等[28]的研究结果一致, 这也解释了pH为9和10时生物炭磷吸附量发生较大降低原因。但pH在3—10, CSLa吸附后的溶液pH均高于CS400, 这是因为镧改性增加了生物炭表面的羟基[29]。
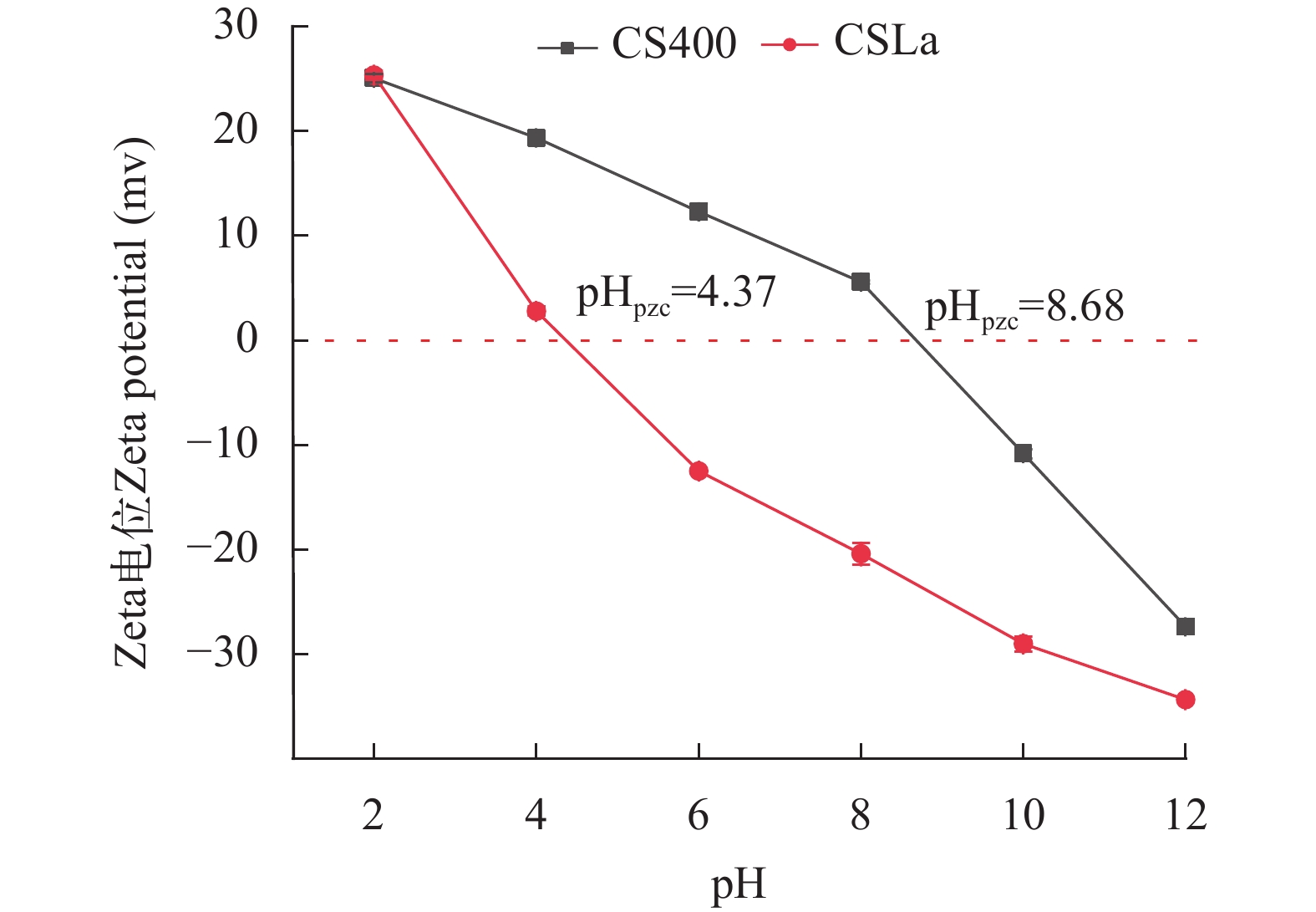
由图 7可知镧改性后, 虾壳生物炭的零点电荷由pH=4.37迁移至pH=8.68, 说明CSLa的电负性弱于CS400[26], 也就是说镧的改性使得CSLa表面正电荷增多, 这可能是因为生物炭表面形成了镧的金属氧化物[26]。CSLa表面正电荷的增多不可避免将于溶液中的磷酸根离子发生更强的静电吸附作用。但是在强碱性磷酸盐溶液中, CSLa的电负性极强, 这种情况是不利于磷酸盐酸吸附的, 这与pH对磷酸盐的吸附性影响结果相一致, 可知静电吸附是CSLa对磷酸盐的主要吸附机理。
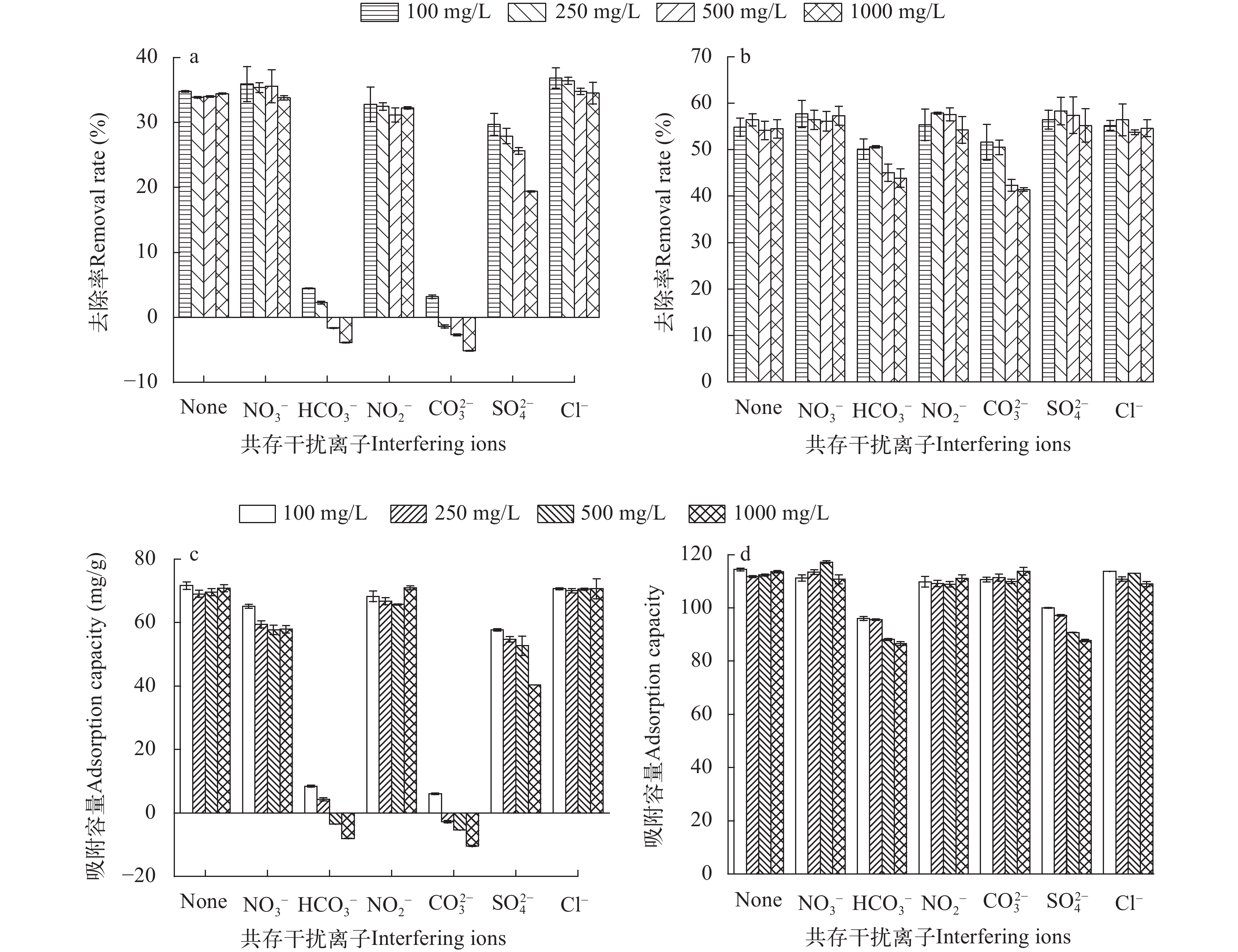
干扰离子的影响 图 8可知
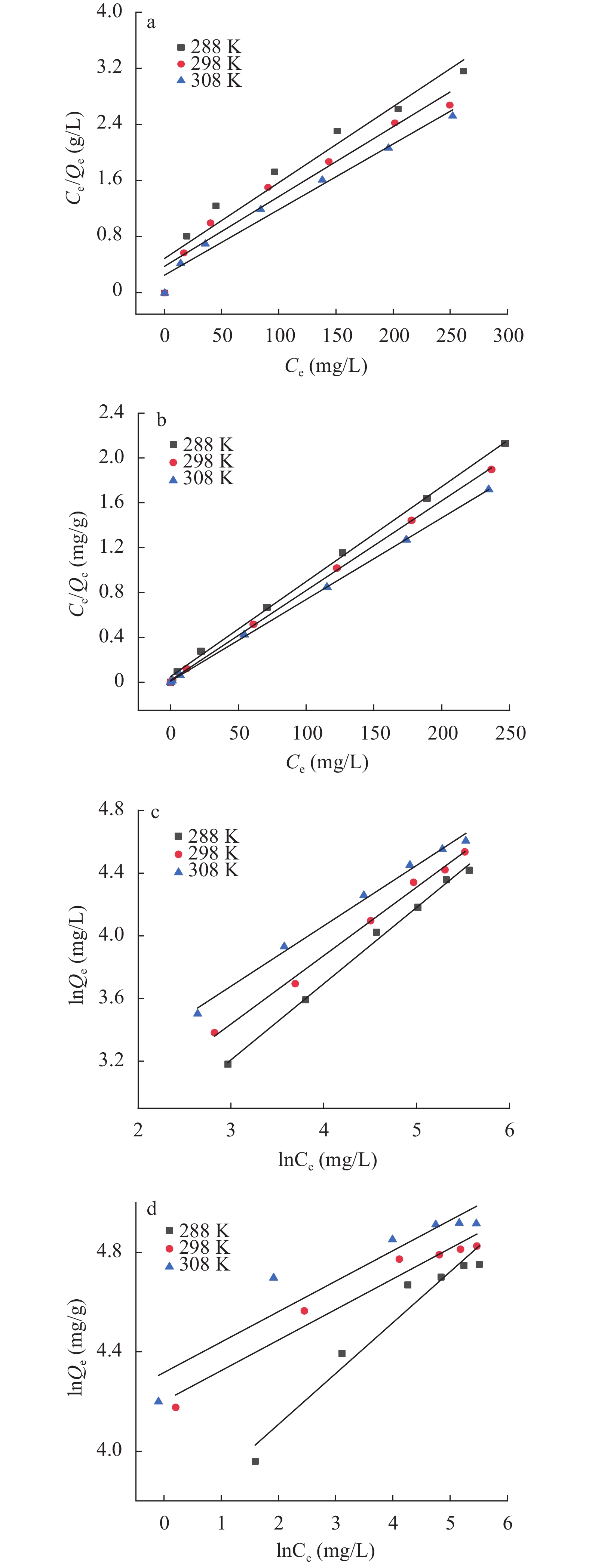
${\rm{NO}}^-_3 $ 、${\rm{NO}}^-_2 $ 、${\rm{Cl}}^- $ 三种阴离子对于CS400/CSLa吸附磷酸盐几乎没有影响,${\rm{SO}}^{2-}_4 $ 对CS400吸附磷酸盐有一定的影响, 推测可能是${\rm{SO}}^{2-}_4 $ 与CS400表面的Ca2+反应生成硫酸钙属于微溶物, 吸附在生物炭表面导致堵塞生物炭的孔径, 影响生物炭对磷酸盐的吸附[30]。${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-}$ 对生物炭吸附磷酸盐的干扰作用明显, 随着${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-} $ 浓度的增加, 生物炭对磷酸盐的吸附逐渐降低甚至释放生物炭含有的磷酸盐。${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-} $ 中存在水解而使吸附溶液pH增加, 导致磷酸盐和生物炭表面的静电吸附和配体交换作用减弱。当${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-} $ 浓度较高时, 不仅抑制生物炭与磷酸盐的静电吸附和配体交换, 同时${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-} $ 还可以与生物炭表面的Ca2+反应, 生成CaCO3沉淀, 附着在生物碳表面及堵塞孔隙, 从而抑制磷酸盐的吸附。${\rm{CO}}^{2-}_3 $ 和${\rm{HCO}}^{3-} $ 浓度过高时, 生物炭中的含磷化合物溶解, 导致生物炭释放内源的磷酸盐。此外,${\rm{HCO}}^{3-} $ 可与Ca2+形成内球配合物, 阻碍了Ca-P的形成[31]${\rm{HCO}}^{3-} $ 和${\rm{CO}}^{2-}_3 $ 两种阴离子均对CSLa起到一定程度的竞争吸附作用, 其去除率和吸附量均有所降低, 但吸附量仍能达到86 mg/g以上, 说明CSLa对磷酸盐有较好的吸附选择性。${\rm{HCO}}^{3-} $ 和${\rm{CO}}^{2-}_3 $ 对生物炭CSLa吸附磷酸盐的抑制作用可能是${\rm{HCO}}^{3-} $ 和${\rm{CO}}^{2-}_3 $ 与CSLa上La3+反应形成了La2 (CO3)3, 而La2 (CO)3的Ksp(3.98×10–34)值小于LaPO4的Ksp值(3.7×10–23)更容易形成沉淀, 所以对磷酸盐形成竞争吸附作用。此外,${\rm{HCO}}^{3-} $ 和${\rm{CO}}^{2-}_3 $ 在水溶液中水解, 使得溶液pH升高, 不利于CSLa吸附磷酸盐。吸附等温线 由图 9和表 3可知CSLa对磷酸盐的吸附可以通过Langmuir吸附等温线模型进行解释, 即CSLa吸附磷属于单层吸附, 而CS400与CSLa相反, CS400更适合用Feundlich吸附等温线模型解释磷酸盐吸附行为, 即CS400吸附磷属于多层吸附。最大理论吸附量为160.51 mg/g, 与CS400磷最大吸附量(100.60 mg/g)相比约提高60%。
表 3 CS400和CSLA吸附等温线模型参数Table 3. Adsorption isotherm model parameters by CS400 and CSLA项目Item Langmuir model Freundlich model Temperature (K) k1 (L/mg) Qm (mg/g) R2 kf (L/mg) n R2 CS400 288 0.02 92.51 0.9412 1.75 0.49 0.9957 298 0.04 107.07 0.9505 2.13 0.44 0.9950 308 0.03 100.60 0.9771 2.52 0.39 0.9929 CSLa 288 0.16 118.06 0.9987 4.89 0.02 0.9520 298 0.17 144.30 0.9947 6.68 0.02 0.9932 308 0.26 160.51 0.9967 6.80 0.01 0.9235 Freundlich等温线中的kf可以用来表示吸附量的多少, 通过表 3可知, 在所设计的研究温度范围内, kf随着温度升高而增大, 也就说生物炭对磷酸盐的吸附效果随着温度的升高而增加, 这可能是温度的升高可以使反应中的活化分子数量增加, 进一步增强了生物炭对磷酸盐的吸附。
Freundlich等温线模型中的通用常数n可以来描述磷酸盐吸附强度[32, 33], CS400和CSLa的两种生物炭的拟合后的n均小于1, 也就说明生物炭对磷酸盐的吸附过程属于有利吸附。
图 10不同初始磷浓度条件下CS400和CSLa对磷酸盐的吸附效果可知, 在低浓度磷酸盐溶液下CSLa对磷的吸附量和去除率均高于CS400。
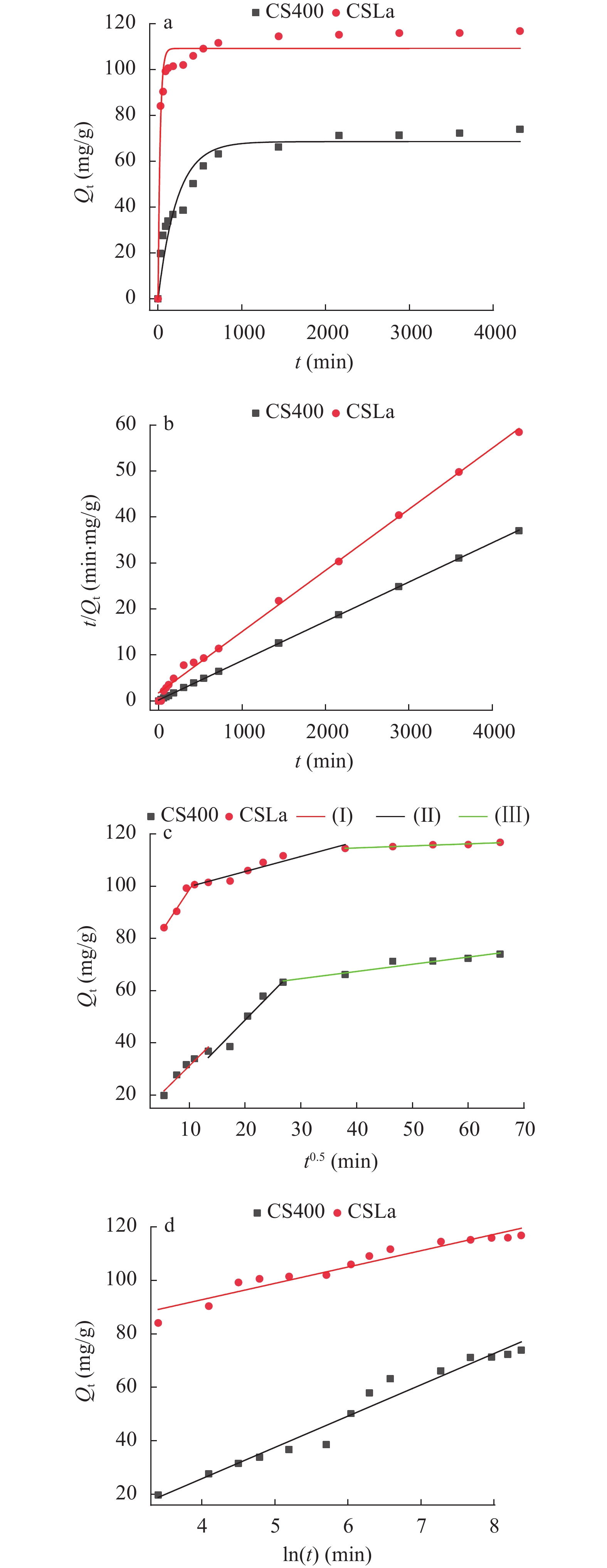
吸附动力学 CS400和CSLa的吸附动力学更加符合二级动力学模型, 依据二级动力学模型属性, CS400和CSLa吸附磷酸盐最主要的速率控制步骤为化学吸附[32, 33]。由一级动力学模型可看出镧改性使生物炭对磷的吸附由原本的12h基本达到饱和提升到1.5h左右, 且吸附量在所设置浓度条件下, 提升了将近60%, 可看出镧改性虾壳所制备的生物炭能高效吸附磷(表 4)。
表 4 CS400和CSLa吸附磷酸盐的动力学模型参数Table 4. Kinetic parameters of phosphorus adsorption by CS400 and CSLaPseudo first-order model Pseudo-second-order model k1 (/min) Qm (mg/g) R2 k2 (min) Qm [g(mg·
min)]R2 CS400 0.0044 68.55 0.9079 0.0001 75.19 0.9971 CSLa 0.0396 109.20 0.9503 0.0003 116.96 0.9999 Elovich Intraparticle diffusivity (Ⅰ) α [mg/
(g·min)]β (mg/g) R2 kw1 (/min) b R2 CS400 1.96 0.09 0.9645 2.17 10.07 0.9388 CSLa 445631.56 0.16 0.9325 0.08 111.56 0.9589 Intraparticle diffusivity (Ⅱ) Intraparticle diffusivity (Ⅲ) kw2 (/min) b R2 kw3 (/min) b R2 CS400 2.18 5.15 0.9461 0.28 56.32 0.9334 CSLa 0.28 56.32 0.9334 0.26 42.64 0.8991 由图 11c可知CS400和CSLa的Elovich模型相关系数R2, 可知CS400和CSLa符合Elovich吸附模型, 吸附过程存在非均相的表面化学吸附行为[34]。
由图 11d为CS400和CSLa的颗粒内扩散动力学模型, CS400和CSLa的整个吸附过程由三个阶段(Ⅰ、Ⅱ和Ⅲ)组成, 磷由溶液中扩散到CSLa表面速度优于CS400, 而在生物炭内扩散速度却低于CS400, 是因为CSLa表面有更多的磷吸附位点和官能团所致。三阶段都不经过原点, 说明颗粒内扩散不是唯一控制吸附过程的步骤, 还存在颗粒外扩散和液膜扩散的吸附过程的步骤[35, 36]。
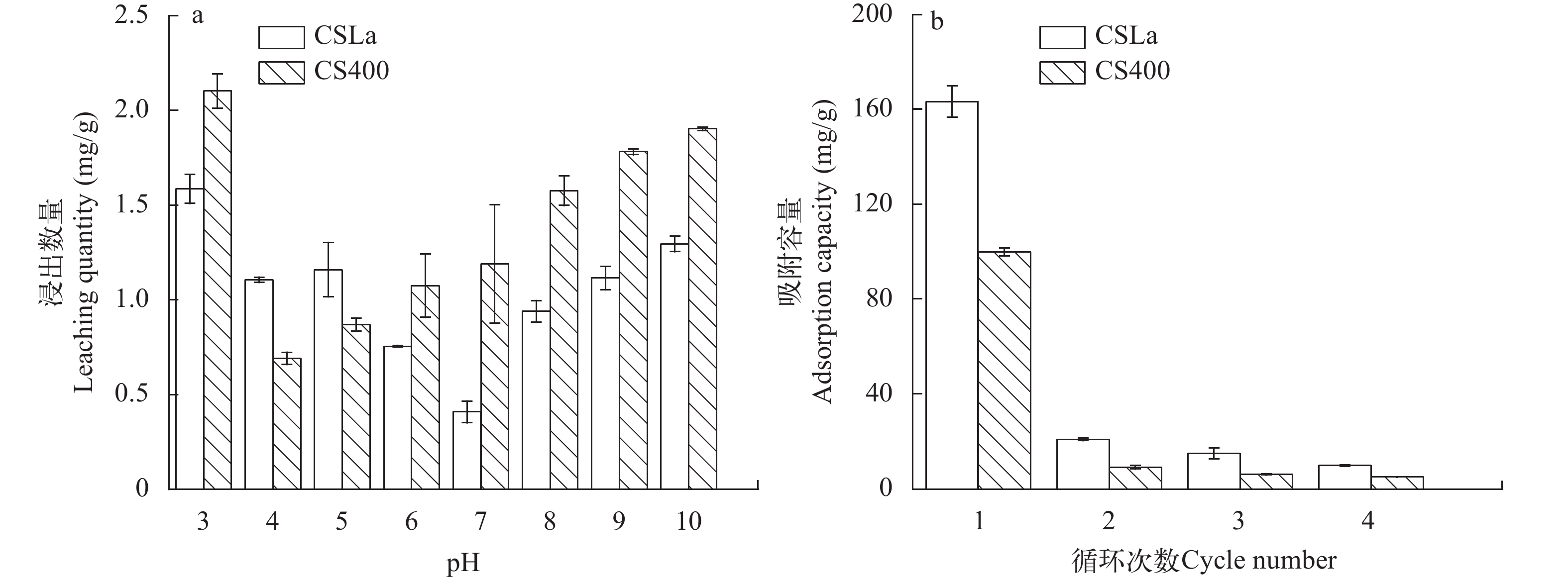
吸附剂的磷释放性能和磷的回收与利用研究 图 12为CS400和CSLa的浸出实验和循环利用实验, 在浸出实验中, 两种生物炭在pH为3—10都存在释放, 随着溶液pH从中性变为酸性或碱性, 生物炭的释放量逐渐增加, 在pH为10和3时释放量最大, 表明生物炭中的磷在弱酸和弱碱条件下存在形式是比较稳定的, 不易在水中进行发生解离。
在循环实验中, 两种生物炭在1 mol/L NaOH再生剂作用下, 初次再生和重复利用对磷酸盐的吸附量都比较低, 表明NaOH不能实现对虾壳生物炭吸附磷酸盐后循环利用, 可考虑将吸附磷酸盐后的生物炭作为缓释肥料, 实现资源化利用。
3. 结论
(1)虾壳生物炭本身对磷具有较高的吸附能力, 最大吸附量100.60 mg/g, 主要是因为虾壳生物炭中的钙相关化合物对磷的吸附起主要作用。(2)镧改性虾壳生物炭具有较强的除磷能力, 最大吸附量160.51 mg/g, 与未改性前相比约提高了60%。主要是因为CSLa中的镧相关化合物对磷的吸附起重要作用。(3)本研究以低温400℃对镧改性进行热解制备生物炭, 改性后其磷吸附量明显提高, 与以往研究相比低温更切合实际, 更利于实际的生产应用。(4) CSLa在较广的pH范围内吸附效果较好, 而CS400更适合在酸性条件下吸附磷酸盐。(5) CSLa吸附过程更符合准二级动力学方程和Langmuir 模型, 表明吸附过程主要为单层的化学吸附。CS400吸附过程更符合准二级动力学方程和Feundlich模型, 表明吸附过程主要为多层的化学吸附。(6) CS400和CSLa的吸附-解吸性能较差, 可考虑将其作为肥料进一步开发利用。
-
表 1 CS400和CSLa的X荧光光谱
Table 1 X-fluorescence spectrum of CS400 and CSLa
组分
Component含量
Content (%)组分
Component含量
Content (%)CS400 CSLa CS400 CSLa La2O3 0.07 76.92 K2O 0.46 0.03 CaO 86.33 16.72 SrO 0.41 0.03 P2O5 5.74 1.28 TiO2 0.08 0.02 SiO2 2.28 0.41 ZnO 0.04 0.02 MgO 1.35 0.15 CuO 0.04 0.02 Fe2O3 0.82 0.25 ZrO2 0.02 / SO3 0.77 0.20 Cr2O3 0.01 / MnO 0.59 0.11 WO3 0.01 / Cl 0.51 2.85 NiO 0.01 0.01 Na2O / 0.40 PbO / 0.02 Al2O3 / 0.19 Tb4O7 / 0.07 Cs2O / 0.11 SrO / 0.03 CeO2 / 0.11 V2O5 / 0.03 表 2 生物炭的BET参数
Table 2 BET parameter of biochar
样品Sample 表面积Specific area (m2/g) 体积Volume (cm3/g) 平均孔径Average pore diameter (nm) CS400 170.06 0.31 7.26 CSLa 151.86 0.30 8.03 表 3 CS400和CSLA吸附等温线模型参数
Table 3 Adsorption isotherm model parameters by CS400 and CSLA
项目Item Langmuir model Freundlich model Temperature (K) k1 (L/mg) Qm (mg/g) R2 kf (L/mg) n R2 CS400 288 0.02 92.51 0.9412 1.75 0.49 0.9957 298 0.04 107.07 0.9505 2.13 0.44 0.9950 308 0.03 100.60 0.9771 2.52 0.39 0.9929 CSLa 288 0.16 118.06 0.9987 4.89 0.02 0.9520 298 0.17 144.30 0.9947 6.68 0.02 0.9932 308 0.26 160.51 0.9967 6.80 0.01 0.9235 表 4 CS400和CSLa吸附磷酸盐的动力学模型参数
Table 4 Kinetic parameters of phosphorus adsorption by CS400 and CSLa
Pseudo first-order model Pseudo-second-order model k1 (/min) Qm (mg/g) R2 k2 (min) Qm [g(mg·
min)]R2 CS400 0.0044 68.55 0.9079 0.0001 75.19 0.9971 CSLa 0.0396 109.20 0.9503 0.0003 116.96 0.9999 Elovich Intraparticle diffusivity (Ⅰ) α [mg/
(g·min)]β (mg/g) R2 kw1 (/min) b R2 CS400 1.96 0.09 0.9645 2.17 10.07 0.9388 CSLa 445631.56 0.16 0.9325 0.08 111.56 0.9589 Intraparticle diffusivity (Ⅱ) Intraparticle diffusivity (Ⅲ) kw2 (/min) b R2 kw3 (/min) b R2 CS400 2.18 5.15 0.9461 0.28 56.32 0.9334 CSLa 0.28 56.32 0.9334 0.26 42.64 0.8991 -
[1] 沈建筑, 李潇轩, 李志辉, 等. 浅析淡水养殖尾水处理技术及达标排放措施 [J]. 水产养殖, 2019, 40(5): 37-39. doi: 10.3969/j.issn.1004-2091.2019.05.014 Shen J Z, Li X X, Li Z H, et al. Analysis of freshwater aquaculture tailwater treatment technology and measures to meet the standard discharge [J]. Journal of Aquaculture, 2019, 40(5): 37-39. doi: 10.3969/j.issn.1004-2091.2019.05.014
[2] 陶玲, 彭格格, 陈思媛, 等. 稻田湿地循环利用池塘养殖尾水效果 [J]. 水生生物学报, 2022, 46(10): 1466-1474. doi: 10.7541/2022.2022.0178 Tao L, Peng G G, Chen S Y, et al. Effect of recycling fishpond wastewater in paddy wetland [J]. Acta Hydrobiologica Sinica, 2022, 46(10): 1466-1474. doi: 10.7541/2022.2022.0178
[3] 曹涛涛, 徐栋, 白国梁, 等. 以RAS固体废弃物为碳源的改进型人工湿地对养殖尾水的脱氮效果 [J]. 水生生物学报, 2022, 46(10): 1475-1483、1591. doi: 10.7541/2023.2022.0190 Cao T T, Xu D, Bai G L, et al. Nitrogen removal effect of aquaculture water through improved constructed wetland using recirculating aquaculture system solid waste as carbon source [J]. Acta Hydrobiologica Sinica, 2022, 46(10): 1475-1483、1591. doi: 10.7541/2023.2022.0190
[4] Genz A, Kornmüller A, Jekel M. Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide [J]. Water Research, 2004, 38(16): 3523-3530. doi: 10.1016/j.watres.2004.06.006
[5] Desmidt E, Ghyselbrecht K, Zhang Y, et al. Global phosphorus scarcity and full-scale P-recovery techniques: a review [J]. Critical Reviews in Environmental Science and Technology, 2015, 45(4): 336-384. doi: 10.1080/10643389.2013.866531
[6] Cordell D, Rosemarin A, Schröder J J, et al. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options [J]. Chemosphere, 2011, 84(6): 747-758. doi: 10.1016/j.chemosphere.2011.02.032
[7] Almanassra I W, Mckay G, Kochkodan V, et al. A state of the art review on phosphate removal from water by biochars [J]. Chemical Engineering Journal, 2021(409): 128211. doi: 10.1016/j.cej.2020.128211
[8] Wu B L, Wan J, Zhang Y Y, et al. Selective phosphate removal from water and wastewater using sorption: process fundamentals and removal mechanisms [J]. Environmental Science & Technology, 2020, 54(1): 50-66.
[9] Chen H B, Gao Y R, Li J H, et al. Engineered biochar for environmental decontamination in aquatic and soil systems: a review [J]. Carbon Research, 2022, 1(1): 1-12. doi: 10.1007/s44246-022-00001-9
[10] Yao Y, Gao B, Chen J J, et al. Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer [J]. Environmental Science & Technology, 2013, 47(15): 8700-8708.
[11] Sornhiran N, Aramrak S, Prakongkep N, et al. Silicate minerals control the potential uses of phosphorus-laden mineral-engineered biochar as phosphorus fertilizers [J]. Biochar, 2022, 4(1): 14-30. doi: 10.1007/s42773-022-00143-4
[12] 廖太万. 含镧化合物改性除磷吸附剂的制备及吸附性能研究 [D]. 广州: 暨南大学, 2019: 19-56. Liao T W. Preparation of lanthanum compound modified adsorbents and studies of their phosphate removal performances [D]. Guangzhou: Jinan University, 2019: 19-56.
[13] 赵莎莎. 镧改性稻壳炭对磷吸附性能研究 [D]. 重庆: 西南大学, 2021: 13-14. Zhao S S. Adsorption performance of phosphate by lanthanum modified rice husk char [D]. Chongqing: Southwest University, 2021: 13-14.
[14] 罗元, 谢坤, 冯弋洋, 等. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能 [J]. 化工进展, 2021, 40(2): 1121-1129. doi: 10.16085/j.issn.1000-6613.2020-0753 Luo Y, Xie K, Feng G Y, et al. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129. doi: 10.16085/j.issn.1000-6613.2020-0753
[15] Wang W W, Zhu S J, Li N H, et al. Enhanced Cd2+ adsorption and toxicity for microbial biofilms in the presence of TiO2 nanoparticles [J]. Environmental Pollution, 2022(314): 120239. doi: 10.1016/j.envpol.2022.120239
[16] Shimizu T, De S K K, Hara M, et al. Facile synthesis of carbon nanotubes and cellulose nanofiber incorporated graphene aerogels for selective organic dye adsorption [J]. Applied Surface Science, 2022(600): 154098. doi: 10.1016/j.apsusc.2022.154098
[17] Mahyoob W, Alakayleh Z, Abu H H A, et al. A novel co-processed olive tree leaves biomass for lead adsorption from contaminated water [J]. Journal of Contaminant Hydrology, 2022(248): 104025. doi: 10.1016/j.jconhyd.2022.104025
[18] Wang J X, Liu P, Huang H J, et al. Pyrolysis of different sewage sludge feedstocks for biochar products: Characterization and application [J]. Journal of Central South University, 2020, 27(11): 3302-3319. doi: 10.1007/s11771-020-4548-y
[19] Diaz D T J L, Silva A M T, Faria J L, et al. Removal of Sudan IV from a simulated biphasic oily wastewater by using lipophilic carbon adsorbents [J]. Chemical Engineering Journal, 2018(347): 963-971. doi: 10.1016/j.cej.2018.04.105
[20] Taffarel S R, Rubio J. On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites [J]. Minerals Engineering, 2009, 22(4): 336-343. doi: 10.1016/j.mineng.2008.09.007
[21] Ho Y S, Mckay G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34(5): 451-465. doi: 10.1016/S0032-9592(98)00112-5
[22] 徐沁媛. 湿地植物载镧生物炭La-CB对磷酸盐的吸附性能研究 [D]. 重庆: 重庆大学, 2019: 33-34. Xu X Y. Study on phosphate adsorption by lanthanum doped wetland plants biochars (La-CB) [D]. Chongqing: Chongqing University, 2019: 33-34.
[23] Koilraj P, Sasaki K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: Batch and column studies [J]. Chemical Engineering Journal, 2017(317): 1059-1068. doi: 10.1016/j.cej.2017.02.075
[24] Kong L C, Tian Y, Li N, et al. Highly-effective phosphate removal from aqueous solutions by calcined nano-porous palygorskite matrix with embedded lanthanum hydroxide [J]. Applied Clay Science, 2018(162): 507-517. doi: 10.1016/j.clay.2018.07.005
[25] Zhang L, Zhou Q, Liu J Y, et al. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber [J]. Chemical Engineering Journal, 2012(185-186): 160-167. doi: 10.1016/j.cej.2012.01.066
[26] Wang Z H, Guo H Y, Shen F, et al. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (
${\rm{NH}}^{+}_4 $ ), nitrate (${\rm{NO}}^-_3 $ ), and phosphate (${\rm{PO}}^{3-}_4 $ ) [J]. Chemosphere, 2015(119): 646-653. doi: 10.1016/j.chemosphere.2014.07.084[27] Peng G F, Jiang S Q, Wang Y X, et al. Synthesis of Mn/Al double oxygen biochar from dewatered sludge for enhancing phosphate removal [J]. Journal of Cleaner Production, 2020(251): 119725. doi: 10.1016/j.jclepro.2019.119725
[28] Chen B L, Chen Z M, Lv S F. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate [J]. Bioresource Technology, 2011, 102(2): 716-723. doi: 10.1016/j.biortech.2010.08.067
[29] Yin X J, Li X, Petropoulos E, et al. Phosphate removal from actual wastewater via La(OH)3-C3N4 adsorption: Performance, mechanisms and applicability [J]. science of the Total Environment, 2022(814): 152791. doi: 10.1016/j.scitotenv.2021.152791
[30] 戴田池. 改性秸秆生物炭吸附水中磷酸盐和四环素效能及机理研究 [D]. 哈尔滨: 哈尔滨工业大学, 2021: 41-42. Dai T C. The adsorption efficiency and mechanism of phosphate and tetracyline in water by modified straw biochar [D]. Haerbin: Harbin Institute of Technology, 2021: 41-42.
[31] Yin H B, Kong M, Fan C X. Batch investigations on P immobilization from wastewaters and sediment using natural calcium rich sepiolite as a reactive material [J]. Water Research, 2013, 47(13): 4247-4258. doi: 10.1016/j.watres.2013.04.044
[32] Wang Z H, Shen D K, Shen F, et al. Phosphate adsorption on lanthanum loaded biochar [J]. Chemosphere, 2016(150): 1-7. doi: 10.1016/j.chemosphere.2016.02.004
[33] Huang W Y, Li D, Liu Z Q, et al. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents [J]. Chemical Engineering Journal, 2014(236): 191-201. doi: 10.1016/j.cej.2013.09.077
[34] Mondal S, Majumder S K. Honeycomb-like porous activated carbon for efficient copper (Ⅱ) adsorption synthesized from natural source: Kinetic study and equilibrium isotherm analysis [J]. Journal of Environmental Chemical Engineering, 2019, 7(4): 103236. doi: 10.1016/j.jece.2019.103236
[35] Wu B L, Fang L P, Fortner J D, et al. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites [J]. Water Research, 2017(126): 179-188. doi: 10.1016/j.watres.2017.09.034
[36] Xie S F, Han X G, Kuang Q, et al. Intense and wavelength-tunable photoluminescence from surface functionalized MgO nanocrystal clusters [J]. Journal of Materials Chemistry, 2011, 21(20): 7263-7268. doi: 10.1039/c1jm10745c
-
期刊类型引用(0)
其他类型引用(2)










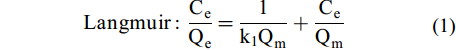
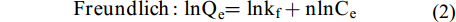
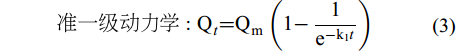
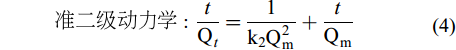
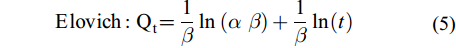
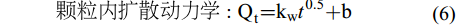
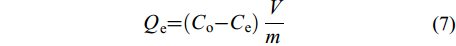
 下载:
下载: